The MHRA Orange Guide
The MHRA Orange Guide
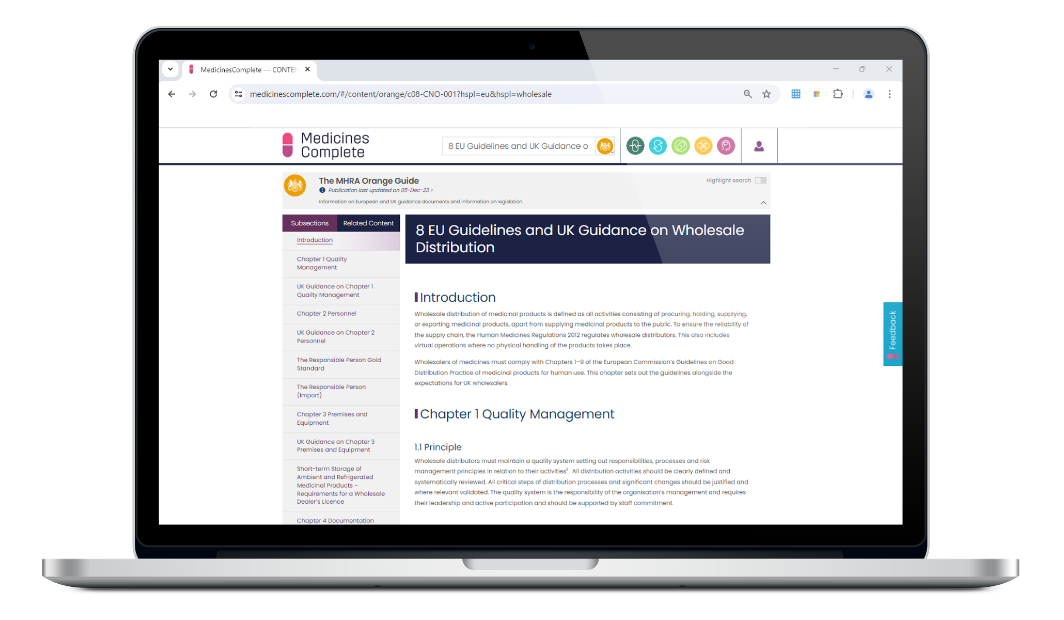

The reference source for manufacturers and distributors of medicines
From Medicines and Healthcare Regulatory Agency (MHRA). Based on current regulation, and regularly updated to reflect changes in best practices, legal requirements, and emerging technologies.
Rules and Guidance for Pharmaceutical Manufacturers and Distributors (The MHRA Orange Guide) is compiled by the Medicines and Healthcare Regulatory Agency (MHRA) as the single source of European and UK guidance and UK legislation on the manufacture and distribution of human medicines, active substances, and brokering medicines.
Supports medicines manufacturers and distributors in meeting legal requirements

The Code of Practice for Qualified Persons

Conditions and guidance for investigational medicinal products

Incorporates changes made after the UK’s exit from the European Union

Minimises research time

Aids good practice

GMP and GDP guidance for active substances

Naming of sites on a licence

Provides a framework for continuous improvement in quality control and monitoring
Request access today
Access The MHRA Orange Guide through MedicinesComplete today.
Contact us now for pricing and access information.
Related resources
Related publications
-
 BEST SELLER
BEST SELLERBNF for Children (BNFC) 2023-2024
The first choice for concise medicines information for children.$99.00
InfoRPS Member Price $49.50 -
 BEST SELLER
BEST SELLERBritish National Formulary (BNF87)
The first choice for concise medicines information Practical and evidence based, British National…$110.00
InfoRPS Member Price $55.00 -
 FEATURED
FEATUREDRules and Guidance for Pharmaceutical Distributions (The MHRA Green Guide) 2022
The reference for medicine distributors in Europe and the UK$103.00
InfoRPS Member Price $77.25
See all our printed publications in the Shop.